Optical tweezers and trapping
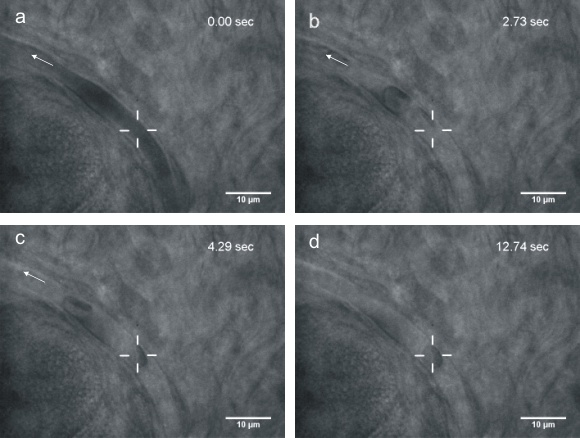
Although microscopic objects can be readily manipulated at the cellular level, additional physiological insight is likely to be gained by manipulation of cells in vivo. Optical tweezers have the advantage to actualize the trapping and manipulation of the biological cells within living animals non-invasively, as the laser is able to penetrate through biological tissues. Here we use infrared optical tweezers to trap and manipulate red blood cells within subdermal capillaries in living mice. We realize a non-contact micro-operation that results in the clearing of a blocked microvessel. Furthermore, we estimate the optical trap stiffness in the capillary. Our work expands the application of optical tweezers to the study of live cell dynamics in animals.

Study of rpoS mRNA with optical tweezers
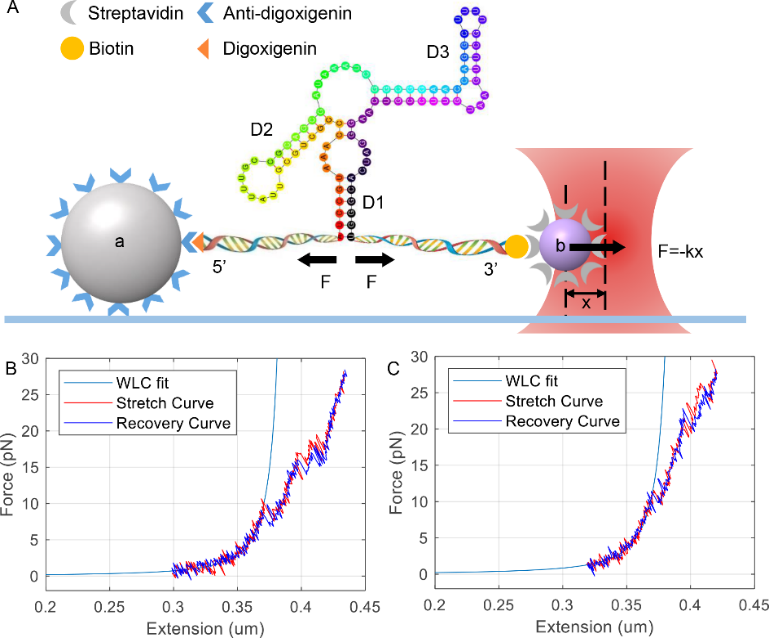
3′ downstream inhibitory stem plays a crucial role in locking rpoS mRNA 5' untranslated region in a self-inhibitory state. Here, we used optical tweezers to study the unfolding/refolding of rpoS inhibitory stem in the absence and presence of Mg2+. We found adding Mg2+ decreased the free energy of the RNA junction without re-arranging its secondary structure, through confirming that this RNA formed a canonical RNA three-way junction. We suspected increased free energy might change the relative orientation of different stems of rpoS and confirmed this by small angle X-ray scattering. Such changed conformation may improve Hfq-bridged annealing between sRNA and rpoS RNA inhibitory stem. We established a convenient route to analyze the changes of RNA conformation and folding dynamics by combining optical tweezers with X-ray scattering methods.
Study of membrane mechanosensation with optical tweezers
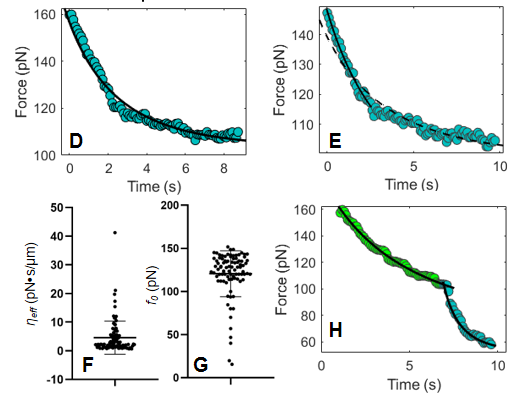
Many cellular processes are orchestrated by dynamic changes in the plasma membrane to form membrane projections and endocytic vesicles in response to extracellular environmental changes. However, there is no quantitative measurement to relate molecular organization of membrane cytoskeletal remodeling to stimulus-elicited mechanosensation on the plasma membrane. Optical tweezers present a powerful tool in the study of membrane tension. Comparing to pulling out an entire membrane tether at one time, the step-like method is more efficient because multiple relaxation curves can be obtained from one membrane tether. Fewer models describe relaxation curves to characterize mechanical properties of cell membrane. Here we establish a new method to measure the membrane relaxation curve of HeLa cells judged by the relationship between membrane tether diameter and tensions. We obtained effective viscosities and static tensions by fitting relaxation curves to our model. We noticed the delicate structure of relaxation curves contains information of cytoskeletal remodeling and lateral protein diffusion. Our study established a quantitative measure to characterize the mechanosensation of epithelial cells in response to stimulus-elicited membrane dynamics.

Advanced optical tweezers
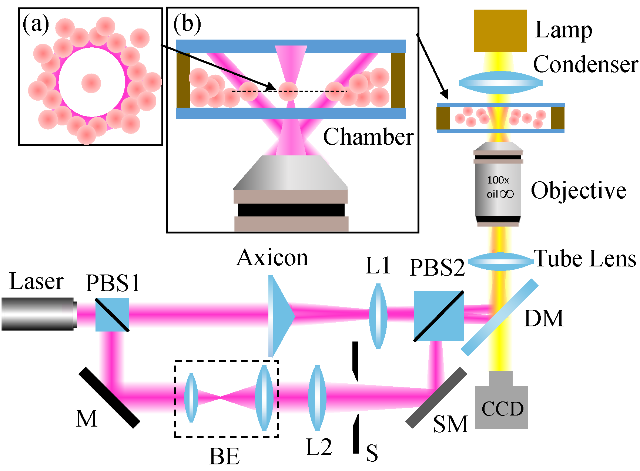
Optical tweezers provide a powerful tool for trapping, guiding, and assembly of biological nanoparticles and cells, which is expected to help people gain physiological insights at single-cell level. However, conventional optical tweezers trap tiny objects using optical gradient forces near a tightly focused Gaussian laser beam, which have a limitation in long-range transportation and manipulation of target objects, and cannot manipulate lots of biological objects. Besides, the Gaussian laser tweezer provides only simple trapping configuration. Worse still, it causes photodamage to the trapped cells. To confront these challenges, we have combined the optical trapping with structured light beams to realize new trapping configurations for complex situations. Furthermore, various types of external fields have been applied for novel tweezers to achieve dynamic manipulation and long-range delivery of target objects. For examples, opto-thermal tweezer, opto-thermoelectric tweezers, and electro-thermos-plasmatic tweezers, have emerged and demonstrated their potentials in cell studies over conventional optical tweezers.
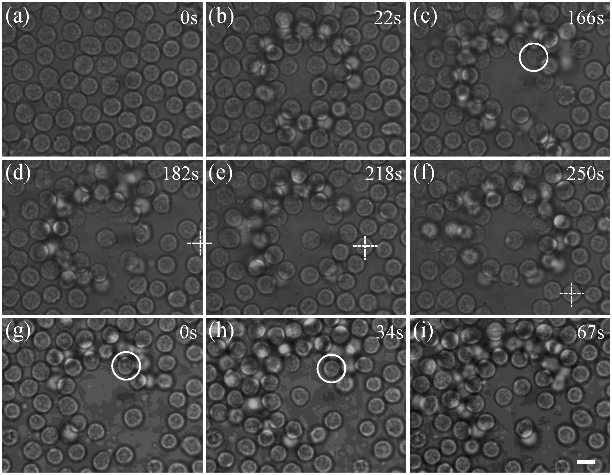
Trapping and manipulating single cells under crowded environments, such as blood vessels and lymph nodes, is still a challenging task. To overcome this issue, an annular beam formed by the far-field Bessel beam is introduced to serve as an optical shield to isolate the target cells from being disturbed. With this scheme, we successfully trapped and manipulated single blood cells in a crowded environment. Furthermore, we demonstrated manipulation of two lymphocytes ejected from a lymph node independently with dual-trap optical tweezers, which paves the way for exploring cell interactions under living conditions.
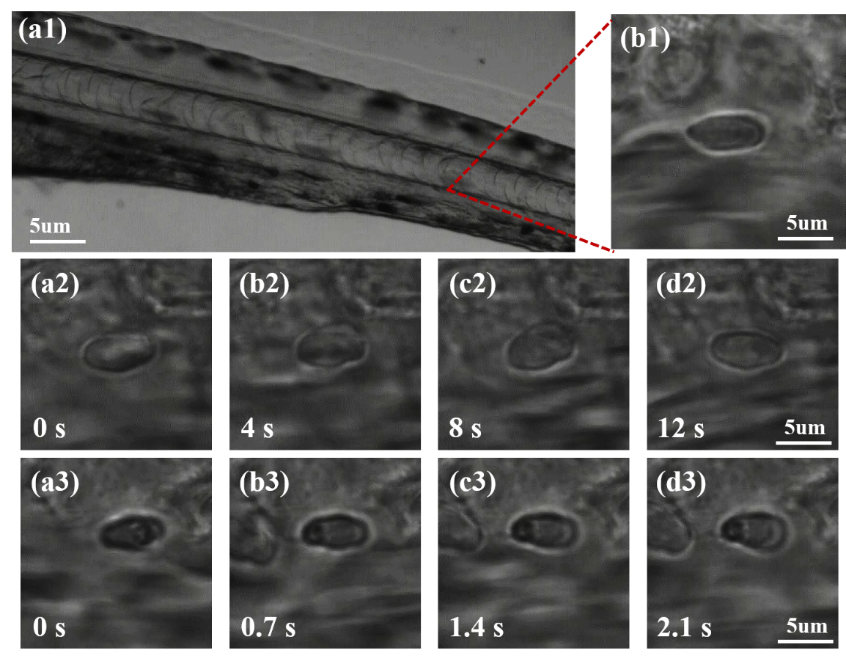
Photodamage is an unavoidable effect in trapping living cells. The effect is obvious for the traditional Gaussian beam trap due to the light intensity concentrates in the center of the highly focused laser beam. We propose to reduce the photodamage in optical trapping of living cells by using azimuthally polarized beams (APBs). APBs achieve higher axial trapping efficiency and lower photodamage than linearly polarized Gaussian beams when used for optical trapping of individual red blood cells (RBCs). In particular, we further achieve reducing photodamage in optical trapping of individual RBCs in living zebrafish.
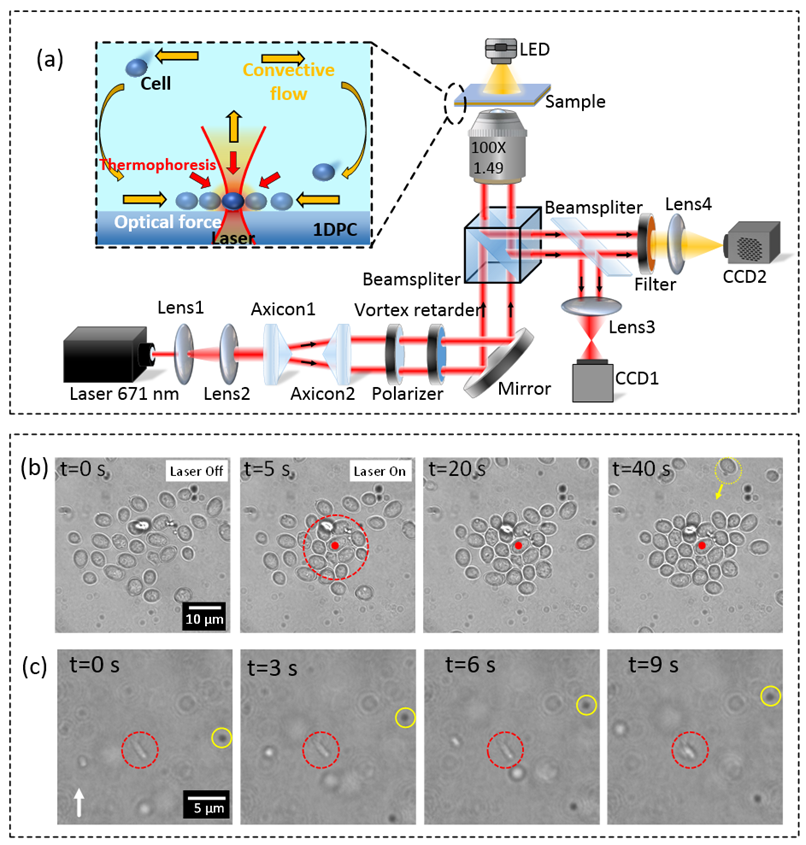
We cooperated with Prof. Douguo Zhang and propose a novel technique for controllable optofluidic assembly of biological cells using an all-dielectric one-dimensional photonic crystal (1DPC) platform. The 1DPC is an all-dielectric multilayer film with a photonic band gap, where Bloch surface waves (BSWs) can be sustained on the top-surface of the multilayer. BSWs feature localized electric field enhancement and will induce local heat source on the multilayer due to the intrinsic loss of the multilayer. Thus, a stable-state temperature gradient field forms on the multilayer. The 1DPC-based tweezer enables the combination of the long-ranged flow-induced force, local thermophoresis force and short-ranged optical force that is associated with focused BSWs. By exploiting the capacity of both optical and thermophoretic manipulation, controllable assembly of many yeast cells as well as E. coli cells have been demonstrated. The optofluidic manipulation with the all-dielectric 1DPC platform opens a new way for long-range transportation, sorting, and self-assembly of biological cells, promoting potential applications in biomedical sciences.
Laser tweezers Raman spectroscopy(Under development)
The laser tweezers enable us to trap and interrogate individual cells, and then collect and analyze the scattered Raman light. Particularly, it allows species such as proteins, nucleic acids, amides and lipids to be identified in an isolated cell that is free from the influence of other cells and extrinsic factors. Furthermore, dynamic changes in cell composition can be observed with higher spatial resolution than obtainable with infrared spectra. The combined features of chemical identification under dynamic conditions with high spatial resolution to follow events at the sub-cellular level without labelling offer great potential for study of single cells or nanoparticles. However, the conventional Raman tweezers used a highly focused laser beam with a high power. Such a configuration causes photodamage to the living cells. This motivated us to look for alternate trapping and tweezing strategies with the following requirements: Low power, single-excitation optical control of reversible nanoassembly with single-molecule SERS sensitivity; Reversible assembly down to the single nanoparticle limit; Reversible assembly that can be achieved within a few seconds. Now we are working on this project.
References:
[1] Trapping red blood cells in living animals using optical tweezers. Nature Communications, 4, 1768 (2013).
[2] Conformation and mechanical property of rpoS mRNA inhibitory stem studied by optical tweezers and X-ray scattering. PLoS ONE 14(9): e0222938 (2019).
[3] Measurement of dynamic membrane mechanosensation using optical tweezers. Journal of Molecular Cell Biology, 2021, 13(6), 455–457.
[4] Trapping and Manipulation of Single Cells in Crowded Environments. Frontiers in Bioengineering and Biotechnology 8, 422 (2020).
[5] Reducing photodamage in optical trapping of individual cells in living zebrafish[J]. Applied Physics Express, 13(3): 032008 (2020).
[6] Controllable optofluidic assembly of biological cells using an all-dielectric one-dimensional photonic crystal. Photonics Research 10, 14 (2022).


